As researchers, we are often eager to get right to the studies, tests, and begin the process of discovery and problem-solving. However ready we may be, research, especially biomedical research comes with critical responsibility – the ethical treatment of human subjects involved in our studies. And, before you even get to informed consent forms for research participants, you must first present your research plan to an Institutional Review Board (IRB).
Many researchers may be initially intimidated by the thought of working with an IRB. The truth is that IRBs are put in place to make sure your study is the best and most ethical it can be. Whether you're a seasoned researcher or a novice stepping into the world of academia, understanding how to effectively collaborate with an IRB is not only essential but can also be an enlightening part of the research process.
An Institutional Review Board (IRB) works to minimize the risks for study participants and ensure those risks are reasonable. The IRB uses a risk versus reward analysis for each study, making sure the dangers are appropriate for the potential reward. They are also in charge of ensuring that the selection of participants is ethical and that informed consent is obtained and properly documented for each participant. The FDA and the Department of Health and Human Services (HHS) also have many monitoring rules in place, so the IRB is tasked with ensuring that the principal investigators can monitor a study’s data. But perhaps their most important job is to ensure the safety of all of the study participants and that their confidentiality is protected.
Every accredited research organization maintains an IRB. A principal investigator must submit a research plan, called a protocol, to an IRB for approval prior to the conduct of the study. The IRB also monitors the study design itself, and guarantees that the research group performs an accurate cost-to-benefit analysis.
What is the Purpose of an IRB and Why is it important?
Institutional Review Boards have been set up in order to maintain rules and ethics in research, especially on humans. Even recent studies in the United States have pushed the boundaries of what is ethical and what is not. For example, the triplet study in the 1960s separated three triplets at birth to test the unsolved question; is nature or nurture a bigger factor in seeing how a child will behave and grow up? This study drew criticism because study director, Peter B. Neubauer, separated the children at birth without telling the biological parents and failing to inform the boys. They only found out when two of the triplets attended the same college; they eventually found the third through a newspaper article. Many academics today believe that the study never would have been allowed to go forth if he worked with an IRB, and the doctor most likely would have been sued for malpractice.
When you are working with an IRB, you should be prepared to answer a multitude of questions about your study. One of the main questions the IRB will ask is whether or not your technology’s contribution to society will be significant enough to be worth whatever risk you are taking. In simpler terms, is this research worth the risk?
Some other common questions you should ask yourself before getting involved with the IRB are: How is my risk measured? Do I need to test vulnerable populations like children or the elderly? Is my study high-risk or minimal-risk? What are the most important practices?
Especially if you’re new to this process, working with an Institutional Review Board can seem daunting and perhaps a bit intimidating. However, if you’re prepared for their questions and have done a deep dive into your own research, by asking important questions, you can make the process a whole lot easier.
Based on experience, here are a few pointers to keep in mind when working with an IRB:
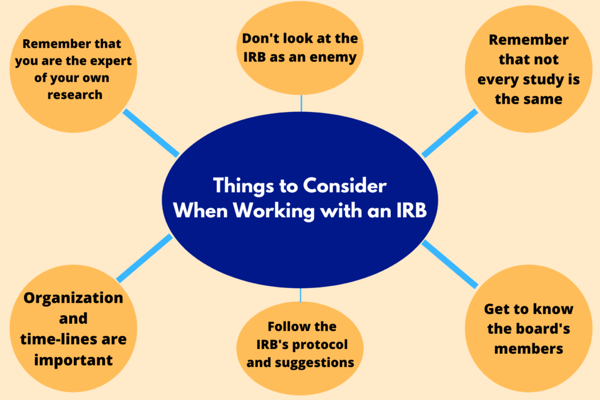
You know your research goal, your purpose, your expected outcome and, if you’ve done the deep dive, all of the moving parts. That being said, you will also need to learn how to communicate that research to others. How will you show others the goals and benefits of what you have set out to test? The IRB needs to understand everything you are trying to accomplish.
Rather than seeing an Institutional Review Board as the enemy, see them as a partner. Their efforts and guidelines are designed to make your work and your research stronger, especially when you present it to a larger scientific community. When meeting with them make sure you take notes and revisit them often. Much like you, they want to see your study succeed, but, they also want it to meet their guidelines. Keep in mind you are not defending your work with them, you are sharing it with them to make it better and safer for all parties.
What may be easy for another research group could be difficult for you. Even if they’re not the same, there will be research out there similar to yours in a variety of ways. Seek out studies with similar work to compare with yours to see what works and what doesn’t. If you find similar past studies it may be worthwhile to contact the study researchers to find out what worked best for them and what they might have changed in dealing with the IRB.
The IRB can tell when a researcher is not organized or not considering their originally drafted timelines. Following timelines is a lot like following protocols and procedures and an inability to adhere to deadlines can impact an IRB’s impression. You want to make sure you stick to your plan, or adjust according to setbacks with open communication and honesty not only with the IRB, but also with yourself.
It is not worth it to do something “your way” when you will most likely have to redo it to the board’s liking anyway. Again, remember to see them as a partner. They’re not working to create obstacles but to strengthen your work.
Using objectivity, keeping in mind human rights, and recognizing the difference between facts and theory will all help you understand whether or not your research will further society in some way.
Getting to know IRB members personally and doing your research will help you when talking with them. You need to know who might take more convincing to support your study and who you know will immediately understand its benefits. None of the members want you to fail, they want you to consider every single aspect of your study. Still, knowing your audience can better help you prepare the information they’d like to see as well as preparing yourself for the questions they may ask.
Data security is the most important job of a protocol. Statistical design can help with data security. A statistical data collection methodology can protect each participant and ensure that data transfer will be safe and ensure confidentiality. Whether it be randomizing data while collecting it or just randomizing subject IDs, there are many ways you can incorporate a statistical methodology in your study. You want to ensure the confidentiality of your participants at all times. Johns Hopkins, for example, offers advice on de-identifying research participant data. However, you can, and should, seek out someone with data, IT, or statistics experience to help randomize and protect your data.
That said, there are additional strategies you can leverage to help protect your participants and their personal data including not collecting any unnecessary data. Believing you “might” need it later, isn’t a good enough reason to collect it. In fact, data you don’t need may just slow you down.

As with any part of your research, rushing any part will show, especially when you need to provide a comprehensive and cohesive presentation of your research. For that reason, spend the time you need when designing your study.
Study design is one of the most important parts of creating a study, especially research involving human participants. While there is no one-size-fits-all when it comes to study design, asking yourself important questions, like the ones that follow, can help you flesh out the details of your research so you’re well prepared for both the IRB and your study.

The answers to these questions might seem overwhelming but it is important to remember the impact a study might have on an individual participant. That is why Institutional Review Boards exist; they want to make sure that your study has an outcome that will help society more than the potential risk you are taking.
Much like an IRB will demand details, thus far we’ve provided an overview of what to expect from working with an IRB, but there are some very specific strategies to help you prepare your study and your presentation to the IRB.
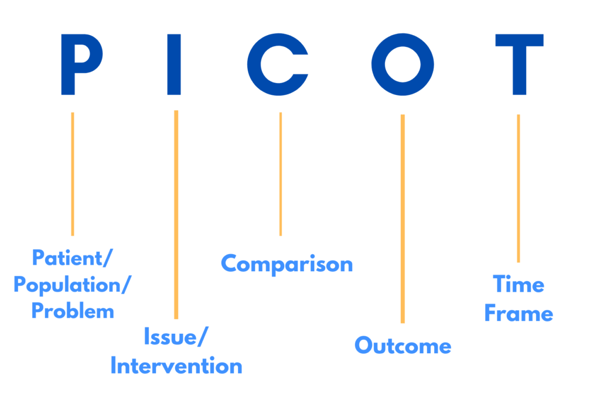
PICOT, for example, is a great acronym to keep in mind when working with an IRB.
P - Patient/Population/Problem. You want the IRB to know the participants are your first priority. Refer to them as people, not as ‘data’, it may make you seem like a ‘Frankenstein doctor’.
I - Issue/Intervention. Make sure you think of confounding variables for your study. If you are a new Primary Investigator (PI) make sure to contact other researchers in the field in order to strengthen your study from the get-go.
C - Comparison. Asking for help from researchers in the same field or PIs from older studies is important to make sure that your study does not repeat past mistakes.
O - Outcome. Brainstorming with teammates will help you envision your outcome more easily. Make sure everyone is on the same page and everyone has a final goal in mind.
T - Time frame. Creating and sticking to a time frame is an important part of every study. Making sure you are organizing and every member of your team is on the same page will help you stick to this time frame.
Sometimes the most important, yet most difficult, part of a study is proving that your particular methods are adequate and that you have enough experience to be able to understand the data. The likelihood that everyone on the IRB is a statistician is low; this means you will need to display the information to convince everyone else as well. From a statistical standpoint, it is important to understand how many variables you will be testing early on. Different methods will apply in each case.
Tests with a single variable may address whether a certain probability model fits the data or whether a population parameter (like the mean) equals a specified value. Tests for two variables address whether there is association between variables or whether one variable is different between groups. You also need to understand whether or not the study variables are categorical or numerical.
Again, remember, part of your goal is to demonstrate your understanding of your research and goals and that means being able to explain it to the full IRB. Thus, as a researcher working with an IRB your goal is to understand your research, communicate it and its goals clearly, and to understand your audience.
As members of an IRB may not be in your exact field, communicating the key elements of your research, including the population you’re working with, your goals, your outcome, and the time frame in which you’ll complete this work must be clear and comprehensive. In short, do your research before presenting your research and you’ll find greater success when working with an Institutional Review Board.
This content comes from a webinar hosted by Dr. Analee Miranda in partnership with ScienceDocs and University Lab Partners. You can watch the full webinar here.
Dr Analee Miranda is an applied mathematician with 15 years of experience. She first learned about statistical methods at the Air Force Research Lab (AFRL) in the Sensors Directorate program, where she worked for 6 years. There, she tested on participants in order to develop a non-Doppler biometric radar system. In recent years, she has been teaching math and statistical work to scientists and engineers.
Be sure to subscribe to our Youtube channel so you will never miss another webinar! Also, connect with us on LinkedIn to see daily updates on University Lab Partners and the SoCal biotech sphere.
Revised 01/03/2024
Download The Ultimate Guide to Wet Lab Incubators in Southern California, a handbook to assist life science start-ups through the entire decision-making process to find wet lab space.
Download Now