Learn more about what you need to receive small business funding.
The 10 tips for writing a competitive SBIR/STTR proposal below will help your company navigate applying for a highly-competitive SBIR/STTR grant. The grant application process can seem complicated and intricate at first, but with the right tools, it can be done efficiently and correctly. This article will help you understand your company's options and your best course of action in order to draft a competitive SBIR or STTR proposal.
🔬Read more about: What are SBIR/STTR Grants?
At the very beginning of your quest for funding, you need to find the best agency for your project (whether it is a device, a drug, a therapeutic, etc). The 2 major places for small businesses that provide funding and deal with biotech innovations are the National Institute of Health (NIH) and the National Science Foundations (NSF). There are many other agencies that set aside money for funding but they are a little bit more ‘picky’ in comparison to the NIH and the NSF. Below are some basic guidelines that will help you draft a competitive proposal.
🔬 Learn about: Funding Options for Biotech Startups
The NIH seeks application of knowledge to enhance health, lengthen life and reduce illness or disability. The NSF on the other hand is more focused on technology and using science and engineering to profoundly impact society. The due dates and other relevant information are detailed in the graphic below. In summary, the NIH works with regular due dates while the NSF works with submission windows in response to the current pandemic. The NIH has said they will be flexible but it is always best to have open communication with the agency if you think you or your company might need an extension.

In the graph above, you will also see that the NIH has a significantly larger budget than the NSF, $1.15 billion in comparison to $1.75 million in Phase I and $110 million in Phase II at the NSF. So this means if your project has an impact in the medical field you might want to consider working with the NIH to maximize your funding. The NSF also requires a 3-page ‘Project Pitch’ that outlines your invention and associated risks. This may seem like just another hoop to jump through, but it is actually a great way to show your innovation to industry professionals at the NSF who can provide valuable insight for your company.
Here is a breakdown of what types of technology or product is best for either the NIH or the NSF.
Another thing to consider when working with the NIH and the NSF is to consider which 'topic area' to apply through. The omnibus/parent grant solicitations at the NIH are generic announcements from the NIH that show that they are looking for a specific type of technology or research. Look at this website to see all of the NIH programs and their specific research. For corresponding information from the NSF, see their website here.
The other type (as opposed to the omnibus/parent solicitations) are called focused solicitations. These are direct calls from agencies for research on a specific topic. Here is a great website that outlines release dates, due dates, and closing dates for specific solicitations.
The two main types of funding mechanisms are the Small Business Innovation Research (SBIR) and the Small Business Technology Transfer (STTR) programs. The table below shows the main differences between the SBIR and the STTR requirements.
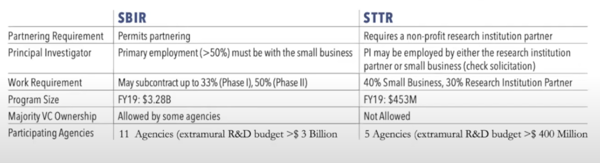
Pay special attention to the work requirements; the NIH has begun to take greater interest in this for small businesses. If you can’t show that your business is involved in the design of the experiments and the interpretation of data you may run into trouble. These agencies want to make sure that your business is involved in the work you claim to do! Also see that the STTR does not allow venture capital relationships.
🔬Learn more: 9 Strategies for Winning Grants at the NIH and Beyond
Getting to know your program officer is one of the most important tips on this list. The Program Officer (PO) in each institute sets a portfolio for the types of topics that they would like to see. The PO will be able to recommend funding to the council. You may be able to get information on other grant applications, so they can help you with better understanding your competition. They will also be able to provide insight on where they think your project is in terms of phases; you can even ask if they think you should fast track your funding mechanism.
A great idea is to discuss your project directly with your PO so you can share your pitch and hear their insights; video conferencing is the best platform because you are able to see their body language in addition to their vocal input. You need to take the initiative on this, the PO will not do it for you! Make sure they know who you are, and if they can’t personally help you it might be worth while to ask for their recommendations for other agencies or applications. Developing a relationship with your PO early on will also help as you continue the application process; they will be able to help you with any questions or concerns you might have. This link will give you the contacts for many program officials at the NIH.
Before you apply for funding from the NIH or the NSF, you need to register your business with their agency. The NIH and the NSF both require you to register your company online and provide a few identification numbers. Trying to register your company at the same time you apply for a grant will be very chaotic, so it is best to do it beforehand. Below is a graphic on what you need to complete before applying for a grant.

Now that you have researched your institute, you want to find out what award you want to apply for. It used to be a simple cross from Phase I to Phase II to Phase III. Now, after Phase II, especially at the NIH, there is a Phase IIB, where you have a product ready to go and you are questioning how to bring it to market. This sub-phase is nicknamed the “Valley of Death’. Instead of immediately being bought out or preparing for marketing, a company in Phase IIB is building funds so that their Phase III trials can be funded by non-SBIR/STTR sources. There is also a way to submit a Phase I and Phase II at the same time, called a fast track at some agencies (more on that later).
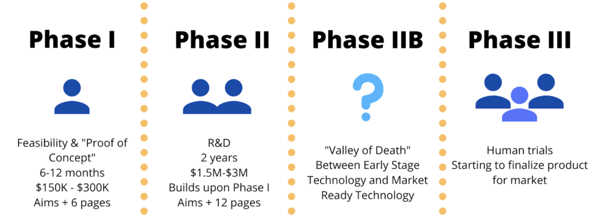
Pay close attention to the page limits and the average times spent in each phase above. Most companies will not be able to move forward into human trials until Phase IIB or Phase III trials. Before then, Phase I and Phase II are mainly rodent models or larger mammals.
Here is a great, and concise, list on the dos and don’ts of phase 1 funding/trials.
The fast track is similar to applying for Phase I and Phase II simultaneously. The advantages are that you can reduce or even eliminate your funding gap. The disadvantage is that the fast track option has a lower success rate and less funds are diverted to this mechanism. The outside perception is that the small business might just be after the money, so be genuine in wanting to get a product or service out there as fast as possible!
Here are a few tips to formulate a competitive Phase II or Fast Track proposal.
The review panel has a very diverse membership; they could be any industry professional including basic scientists, engineers, physicians, surgeons, or past SBIR/STTR awardees. Agencies want the panel to have a unique range of expertise and perspectives. Try to avoid writing for a narrow audience!
To be successful you need many things, including: clearly defined and rigorous experimental strategies with quantifiable endpoints, a clinical need/commercial potential, proof-of-concept data, and a critical experiment approach with discussion of potential pitfalls or alternatives. You also want to include a reasonable timeline for your product or service. There are three reviewers that will read your entire application. Now with the internet most reviewers are easily able to look up content and citations. These 3 reviewers will be the ones to present your application to the entire study section. The rest of the panel will most likely only read your Abstract and Aims Page. You never want to ignore things like possible downfalls or competition on your proposal; the entire panel is able to look up your citations and challenge your findings if the discussion gets ‘heated’.
🔬Read more: Do's & Don'ts of the SBIR Grant Application Process
Below are a few tips to help ease their reviewing process and help draft the most competitive SBIR/STTR proposal:
You should always try to make your applications as straightforward and easy to understand as possible. Creating an inconvenience or an extra hurdle for your reviewers will not help your chances of receiving a good score on your grant application.
These tips for a competitive SBIR proposal come from a webinar from ScienceDocs and University Lab Partners, presented Dr. Pamela Lucchesi. You can watch the full video here.
Dr. Lucchesi received her PhD in Biomedical Sciences from University of Massachusetts Medical School and is an elected fellow of the American Heart Association and the American Physiological Society. She has a 30-year track record of continuous extramural funding related to cardiovascular disease and has mentored numerous fellows, junior faculty, and small business owners.
Download The Ultimate Guide to Wet Lab Incubators in Southern California, a handbook to assist life science start-ups through the entire decision-making process to find wet lab space.
Download Now